Remdesivir Development History
Remdesivir fda approval status.

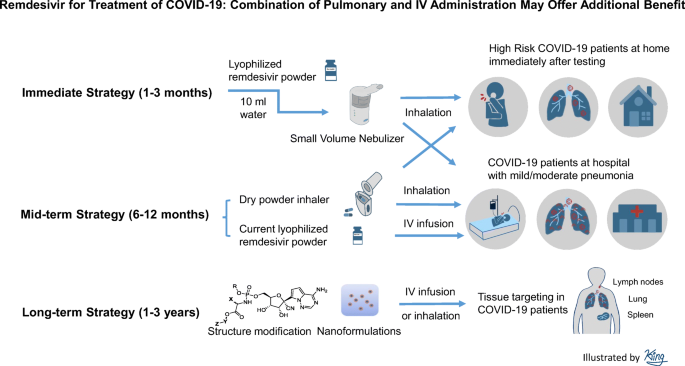
Remdesivir development history. It is administered via injection into a vein. It did not work against hepatitis c as hoped but was then repurposed and studied as a potential treatment for ebola virus disease and marburg virus infections. Remdesivir was originally created and developed by gilead sciences in 2009 as part of the companys research and development program for hepatitis c. Covid 19 remdesivir is an investigational nucleotide analog antiviral in development as a potential treatment for hospitalized patients with severe covid 19.
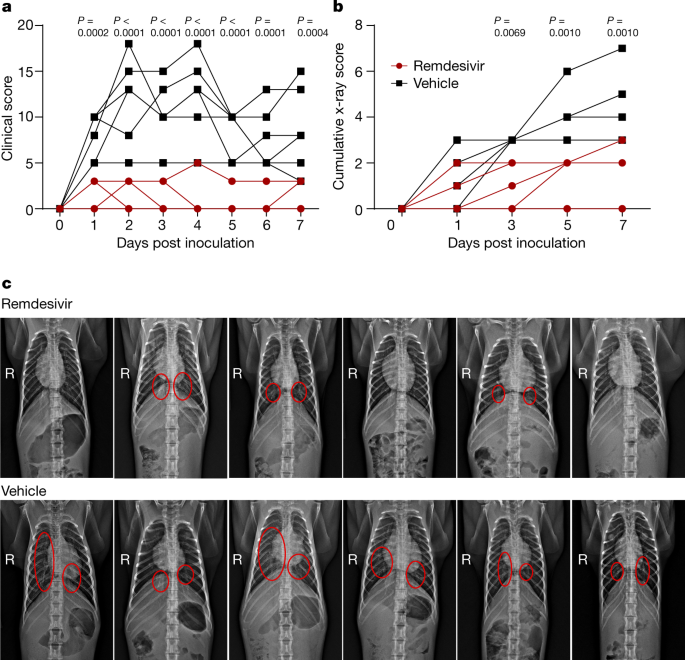
By justin hughes and arti k. Richard whitley md distinguished professor at uab and principal investigator of the u19 grant the investigational drug remdesivir developed through research conducted through the antiviral drug discovery and development center or ad3c and centered at the university of alabama at birmingham is being used to treat select infected patients in the united states and in china who have been. Remdesivir is being tested as a treatment for covid19 and has been authorized for emergency use in the us india singapore and approved for use in japan the european union and australia for. Subsequent evaluation by numerous virology laboratories demonstrated the ability of remdesivir to inhibit coronavirus replication including sars cov 2.
The safety and efficacy of remdesivir for any use have not been determined. The taxpayer drug innovation to the rescue may 2. On august 28 2020 it was announced that the us. Acknowledging the public role in private drug development.
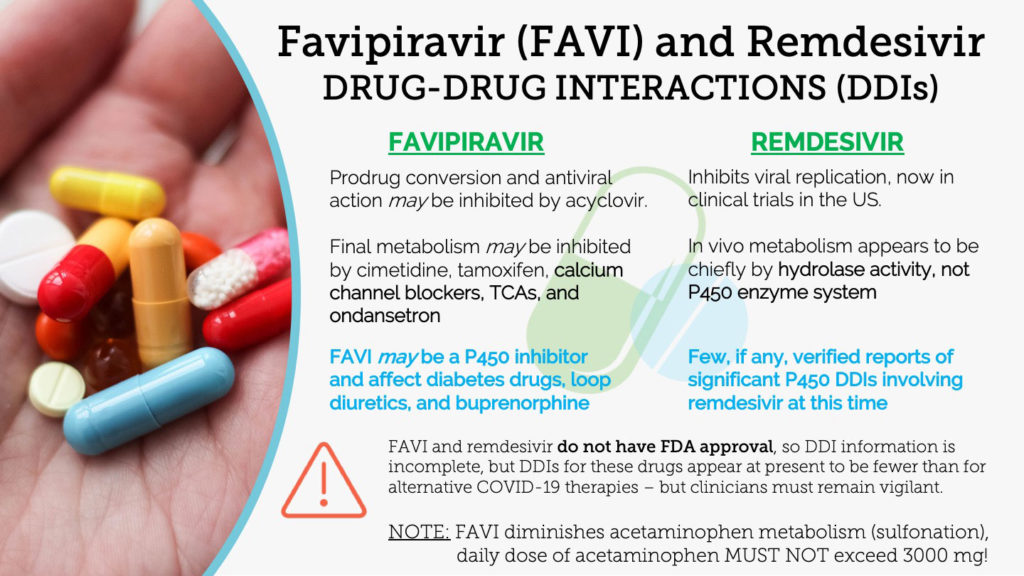
Reviewed by judith stewart bpharmlast updated on aug 11 2020. Although in preclinical development eidd 1931 is orally bioavailable a significant advantage compared to remdesivir and has increased potency against viruses containing mutations in rdrp that conferred increased resistance to remdesivir supporting the potential for a combination therapy to address the risk of sars cov 2 becoming clinically drug resistant. Ebola in 2014 when the ebola outbreak was spreading in west africa gilead scientists believed that antiviral compounds. Development of remdesivir remdesivir is an investigational product and has not been approved anywhere globally.
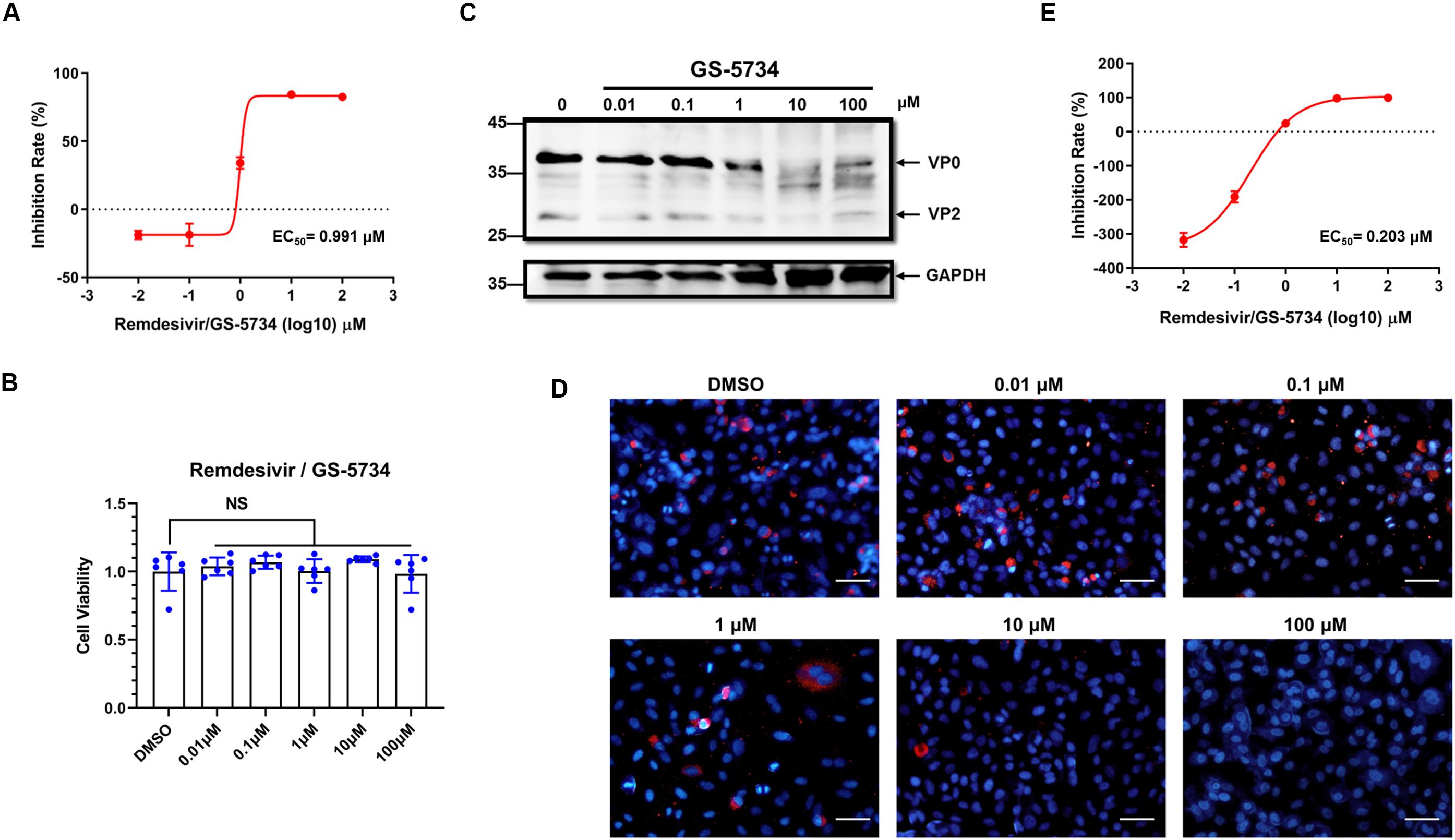
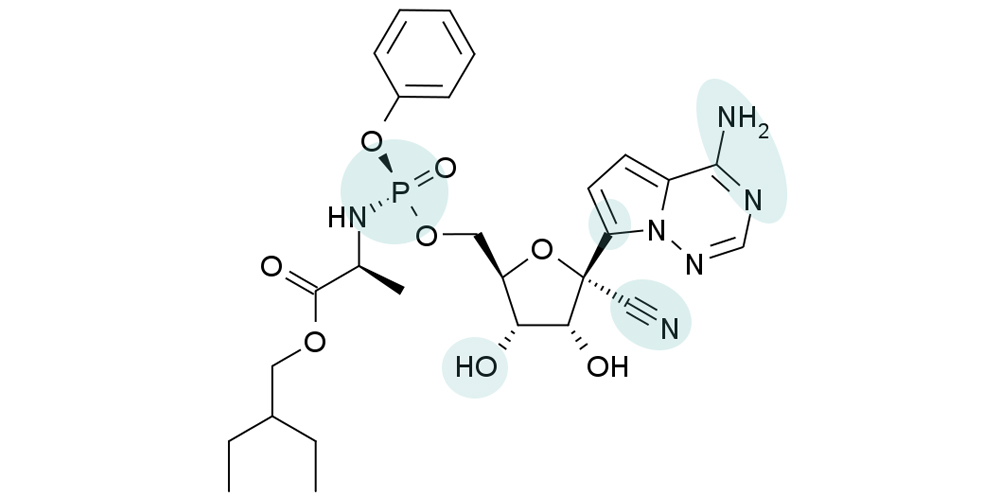
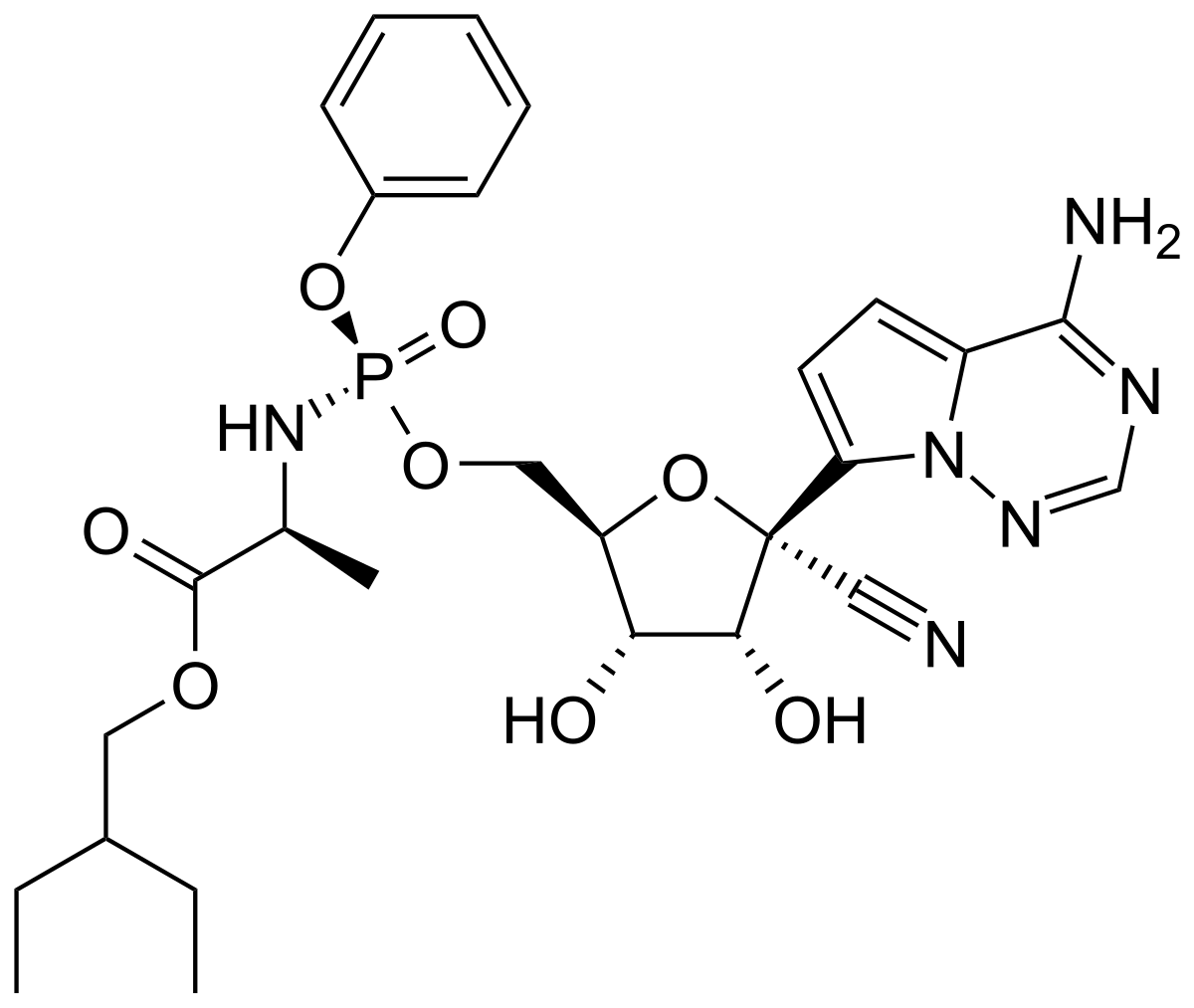
Remdesivir is a nucleotide analogue prodrug that perturbs viral replication originally evaluated in clinical trials to thwart the ebola outbreak in 2014.

Retracted Hydroxychloroquine Or Chloroquine With Or Without A Macrolide For Treatment Of Covid 19 A Multinational Registry Analysis The Lancet
Initial Study Of Remdesivir An Antiviral Drug Shows That Two Thirds Of Severe Covid 19 Patients Got Better Health News Firstpost